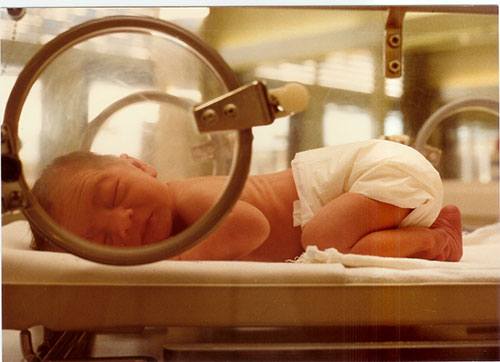
Session Abstract – PMWC 2017 Silicon Valley
Session Synopsis: Up to one-third of US babies who are admitted to a Neonatal Intensive Care Unit have a genetic disease and more than 20% of infant deaths are caused by genetic illnesses. Panelists in this session will discuss the promise of rapid whole-genome testing in acute care situations and the future of the “26-Hour Genome” diagnosis.
Session Chair Profile
Ph.D., President and CEO of Edico Genome
Biography
Pieter has more than 20 years of experience inventing, developing and successfully commercializing technologies in industries including semiconductors, wireless, health care, life sciences, image processing and retail automation, with a portfolio of over 130 granted patents in these areas. His passion for bringing to market innovative technologies has led to the formation and funding of a number of technology start-ups with significant return to investors. Prior to Edico Genome, Pieter was involved in the emerging mobile health industry where mobile phone technology enables innovative health care delivery in underdeveloped communities. Pieter also co-founded ecoATM (acquired in 2013 by Outerwall (NASDAQ:OUTR) for $350 mil.) and Zyray Wireless (acquired in 2004 by Broadcom Corporation (NASDAQ:BRCM) for $100 mil.).
Speaker Profile
BS, MBA, Vice President, Applied Genomics, Illumina, Inc.
Biography
Dawn Barry is the Vice President of Applied Genomics where she leads market development and expansion strategies for early stage clinical, research, and industrial applications of genomics. She was formerly Senior Director of Illumina’s New and Emerging Opportunities business responsible for identifying and catalyzing new markets and solutions. Dawn joined Illumina in 2005 as their first market development specialist focusing on elevating the Company’s presence in the pharmaceutical sector. Since then, Dawn has had similar success bringing Illumina genomic solutions into other markets including agriculture, forensics and cytogenetics. Prior to Illumina, Dawn spent seven years at Genaissance Pharmaceuticals, one of the early genomics start-ups focused on individualized medicine and DNA-based diagnostic testing, with the primary responsibility of promoting clinical genetics testing services for pharmaceutical clinical trials. Dawn holds a Bachelors degree in Biology from the University of Vermont and a Masters of Business Administration from the University of Connecticut School of Business.
Speaker Profile
M.D., Medical Director of Institute for Genomic Medicine, Rady Children’s Hospital – San Diego
Biography
Dr. David Dimmock is a nationally-renowned expert on the field of clinical genomic medicine. Dr. Dimmock joined the Rady Children’s Institute for Genomic Medicine, located in San Diego, CA, in June 2016. He is the clinician primarily responsible for the first use of exome sequencing to change the medical management of a child. This case was the subject of Pulitzer Prize winning articles and the book, One in a Billion: The Story of Nic Volker and the Dawn of Genomic Medicine. In 2010, he was a leader of the team that deployed the first clinical end-to-end whole genome sequencing test. This solution included patient counselling and consent, clinical laboratory testing, data analysis, data return. Before joining Rady Children’s, Dr. Dimmock’s clinical practice focused on the diagnosis of heritable disorders in children and adults and the long term care of patients with mitochondrial and metabolic disorders. He has been the principal investigator for multiple industry sponsored studies evaluating novel therapeutics for these disorders.