VC activity is booming in healthcare, with second quarter drawing in $5.1 billion in capital. That is 22% of the total $23 billion raised by all VC-based companies in the U.S., according to latest Pricewaterhouse Coopers quarterly report.
Some of the highlights include:
- Investments hit record-breaking numbers
- VC-backed U.S. companies raising 45 mega-rounds (totaling more than $100 million each), up from 35 last quarter
- 2018 is currently on pace to beat what was an unprecedented 2017, which posted 111 mega-rounds
- Two of the seven biggest mega-rounds in Q2 were precision medicine companies
- Allogene Therapeutics – commercializing off-the-shelf CAR-T therapies
- GRAIL – clinical-stage early detection of cancer
- Each raising $300 million in oversubscribed financing
- A review by Silicon Valley Bank found that biopharma Series A investments are exploding, and by mid-year reached $2.6 billion – compared to the full-year total of $2.3 billion in 2017
- Underscoring robust pre-IPO biopharma valuations, step-ups from Series A to B have seen a two to three-fold increases in the past 18 months
- Major investments in synthetic biology building blocks, including computational design, CRISPR editing, DNA/RNA synthesis and organism engineering tools, have climbed both in 2017 and 2018
- 75 VC-backed biopharma unicorns have reached valuations of $1 billion or higher since 2013 – either through acquisition or in the public markets
- 30 biopharma companies went public already this year – compared to 31 IPOs for all of 2017
2018 is shaping up to be another strong year in healthcare. In the first six months, biopharma exits have created the momentum for strong fundraising and investment.
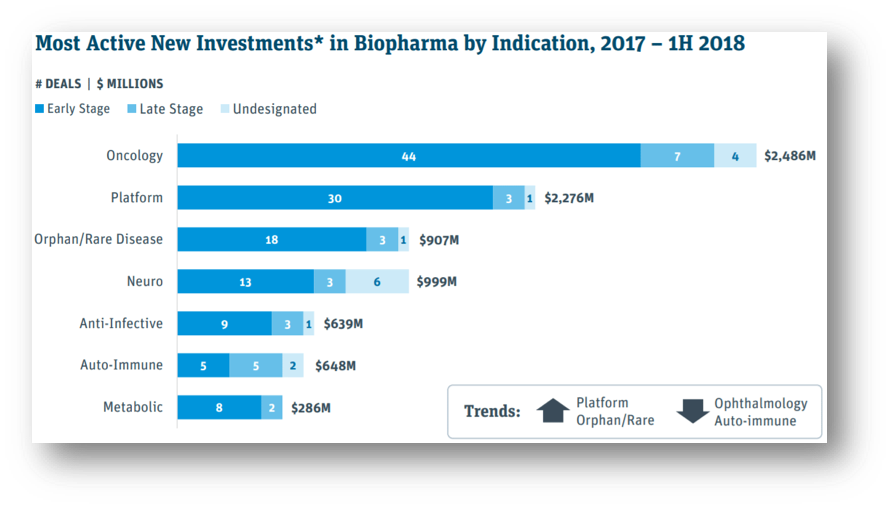
Source: Silicon Valley Bank
*Most Active New Investments in biopharma defined as Top 60 venture and corporate investors based on new investments in 2016–2017
Underscoring the recent influx of capital over the past two years from the most active investors, every top indication in 1H 2018 other than oncology has already surpassed investment levels for full-year 2016. Waves of new biopharma investment are pushing funding for platform companies to levels similar to oncology and driving a comeback for orphan/rare drugs.
The timely PMWC 2018 Duke conference has several exciting sessions scheduled that focus on the investment sector, new technologies, and a platform for rising startups to share their innovative ideas with the audience via the Showcase Track.
Mike Pellini (Section 32)
Dr. Pellini is Managing Partner of Section 32, a venture fund that invests in companies and inventors that are changing the way humans use technology and the way technology betters humanity. Previously, he served as CEO of Foundation Medicine.
Read his full bio and a recently conducted interview with Mike Pellini.
Arthur Pappas (Pappas Ventures)
Art founded Pappas Capital in 1994, and over the past twenty-four years the firm has managed more than $535 million in capital and invested in more than 70 companies. Art currently serves as a director for Aura Biosciences and OrphoMed, and as a board observer for Amplyx Pharmaceuticals, Balance Therapeutics, Kezar Life Sciences and Real Endpoints. Read his full bio.
Dr. Mickey Kertesz (Karius Dx)
Dr. Kertesz will discuss the technology of his latest company that develops novel NGS infectious disease diagnostics methodology that detects microbial cell-free DNA in plasma from more than a thousand pathogens including bacteria. Read his full bio.
Dr. Robert S. Langer (MIT)
Dr. Langer’s major focus area is Nanotechnology. He is developing new nanoparticles to treat cancer and other diseases. Read his full bio.
Dawn Barry (LunaDNA)
Dawn Barry will dive into blockchain and how they unite personal privacy and control around genomic data with a mechanism for rewards for data sharing in research. Read her full bio.
Showcase Track
The showcase track provides a platform for funded, emerging companies to present their innovative new technologies that intend to disrupt healthcare. Showcases include:
- AI and Data Sciences
- Clinical Dx
- Immunotherapy
- Genomic profiling
- Microbial profiling
- Clinical and Research Tools
- Non-clinical services
- Emerging Therapeutics
Apply to present here
Come see these speakers and the Showcase track at
PMWC Duke Sept. 24-25
The Precision Medicine World Conference (PMWC), in its 17th installment, will take place in the Santa Clara Convention Center (Silicon Valley) on January 21-24, 2020. The program will traverse innovative technologies, thriving initiatives, and clinical case studies that enable the translation of precision medicine into direct improvements in health care. Conference attendees will have an opportunity to learn first-hand about the latest developments and advancements in precision medicine and cutting-edge new strategies and solutions that are changing how patients are treated.
See 2019 Agenda highlights:
- Five tracks will showcase sessions on the latest advancements in precision medicine which include, but are not limited to:
- AI & Data Science Showcase
- Clinical & Research Tools Showcase
- Clinical Dx Showcase
- Creating Clinical Value with Liquid Biopsy ctDNA, etc.
- Digital Health/Health and Wellness
- Digital Phenotyping
- Diversity in Precision Medicine
- Drug Development (PPPs)
- Early Days of Life Sequencing
- Emerging Technologies in PM
- Emerging Therapeutic Showcase
- FDA Efforts to Accelerate PM
- Gene Editing
- Genomic Profiling Showcase
- Immunotherapy Sessions & Showcase
- Implementation into Health Care Delivery
- Large Scale Bio-data Resources to Support Drug Development (PPPs)
- Microbial Profiling Showcase
- Microbiome
- Neoantigens
- Next-Gen. Workforce of PM
- Non-Clinical Services Showcase
- Pharmacogenomics
- Point-of Care Dx Platform
- Precision Public Health
- Rare Disease Diagnosis
- Resilience
- Robust Clinical Decision Support Tools
- Wellness and Aging Showcase
See 2019 Agenda highlights:
- Five tracks will showcase sessions on the latest advancements in precision medicine which include, but are not limited to:
- AI & Data Science Showcase
- Clinical & Research Tools Showcase
- Clinical Dx Showcase
- Creating Clinical Value with Liquid Biopsy ctDNA, etc.
- Digital Health/Health and Wellness
- Digital Phenotyping
- Diversity in Precision Medicine
- Drug Development (PPPs)
- Early Days of Life Sequencing
- Emerging Technologies in PM
- Emerging Therapeutic Showcase
- FDA Efforts to Accelerate PM
- Gene Editing / CRISPR
- Genomic Profiling Showcase
- Immunotherapy Sessions & Showcase
- Implementation into Health Care Delivery
- Large Scale Bio-data Resources to Support Drug Development (PPPs)
- Microbial Profiling Showcase
- Microbiome
- Neoantigens
- Next-Gen. Workforce of PM
- Non-Clinical Services Showcase
- Pharmacogenomics
- Point-of Care Dx Platform
- Precision Public Health
- Rare Disease Diagnosis
- Resilience
- Robust Clinical Decision Support Tools
- Wellness and Aging Showcase
- A lineup of 450+ highly regarded speakers featuring pioneering researchers and authorities across the healthcare and biotechnology sectors
- Luminary and Pioneer Awards, honoring individuals who contributed, and continue to contribute, to the field of Precision Medicine
- 2000+ multidisciplinary attendees, from across the entire spectrum of healthcare, representing different types of companies, technologies, and medical centers with leadership roles in precision medicine












